The AMPEL-CDSS is a clinical decision support system in routine care. We focus on increased patient safety and improved diagnostics by harnessing the potential of digital medicine.


This project was funded with tax revenues based on the budget adopted by the members of the Saxon State Parliament (eHealthSax 2018-2022).
Project
Through the AMPEL-CDSS, medical decision-makers are supported in their work to provide patient care through improved availability and automatic prioritisation of medical information.

December
Funding, €2.6 million from public funds in accordance with the budget passed by the Saxon state parliament (eHealthSax), 2018-2022
January
Start AMPEL project
December
CDSS activation, lactatemia
November
Participation MEDICA, Düsseldorf
September
Prize, Annual Meeting of the German Society for Laboratory Medicine, Magdeburg
August
CDSS activation, hypercalcemia
March
CDSS activation, hypokalemia
January
Prize, Life Sciences Research Festival, University of Leipzig
November
New cooperation, Cardiology, Leipzig University Hospital
Award, MSD Health Award 2020
October
New cooperation, Transfusion Medicine, Leipzig University Hospital
February
CDSS Activation, Acute Kidney Injury
CDSS Activation, Hyponatriemia
January
Participation, Research Festival Life Sciences, University of Leipzig
December
CDSS activation, anemia
Roll Out, Department of Surgery, Leipzig University Hospital
November
New cooperation, Endocrinology, University Hospital Leipzig
October
CDSS activation, refeeding syndrome
September
New cooperation, Nutritional Medicine Team, Leipzig University Hospital
April
Roll Out, Department of Internal Medicine, Leipzig University Hospital
December
New cooperation, Pharmacy & Infectiology, Leipzig University Hospital
October
Award, Academy for Patient Blood Management 2022
Funding, 0.4 million Euro by the German government (KHZG 2022-2024)
Participation, Congress of the German Society for Clinical Chemistry and Laboratory Medicine, Mannheim, Germany
September
Award, SAP Innovation Award 2022
May
Award, Congress of the German Society for Internal Medicine, Wiesbaden
Participation, project workshop: “Digitalization in the health care industry” Saxony Economic Development Corporation, online
Participation, Action Day “Laboratory Diagnostics” (BDL & DGKL), Berlin
March
Participation, German Congress of Endocrinology, online
January
New cooperation, anesthesia, Leipzig University Hospital
Ensuring the necessary medical consistency through algorithm-based quality assurance
The core element of the project is the reporting system, which alerts within seconds if there are indications of acute clinical patterns and delays in further diagnosis and treatment. The basis for this is a complex data combination of laboratory medical results and data from the hospital information system.
Starting in 2020, the software was activated at three hospitals. In addition to the tertiary care center, Leipzig University Hospital, two standard care hospitals, the Muldentalkliniken in Grimma and Wurzen, and thus a total of more than 1500 beds are connected to the AMPEL-CDSS. Thanks to the randomized implementation, important scientific evidence on the benefits of clinical decision support systems (CDSS) can be collected.
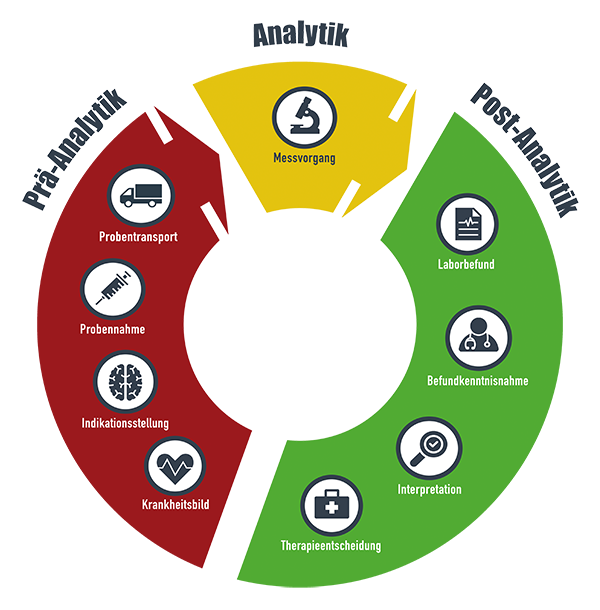
Goals
Consortium

The Institute of Laboratory Medicine, Clinical Chemistry and Molecular Diagnostics (ILM) at Leipzig University Medical Center (UML) is one of the leading laboratory medicine institutes in Germany. Within the scope of patient care, around 1,500 patient examinations with a total of around 20,000 laboratory-medical analyses are carried out daily for the clinic, faculty and the Medical Care Center (MedVZ) of the UKL. The ILM is accredited according to Directive 98/79/EC and DIN EN ISO/IEC 17025, as well as Directives 93/42/EEC, 90/385/EEC and DIN EN ISO 15189. The range of services includes clinical chemistry, hematological diagnostics, hemostaseology, immune and cerebrospinal fluid diagnostics, endocrinological diagnostics as well as molecular and special diagnostics.
In addition, as the Screening Center Saxony, the Institute, together with the Dresden site, provides about 50,000 newborn screening examinations for inborn errors of metabolism annually for the Free State of Saxony and Thuringia. Research at the ILM includes metabolome and protein analysis, as well as molecular and genetic research into vascular diseases and metabolic disorders. The institute also has its own department for the performance of laboratory analytics of clinical studies. There are research collaborations with numerous national and international cooperation partners from research and science. In this context, the ILM also acts as an important partner for major study projects such as the LIFE study (Leipzig Research Center for Civilization Diseases) and the medical informatics initiative of the German Federal Ministry of Education and Research SMITH (Smart Medical Information Technology for Healthcare).

Leipzig University Hospital (UKL), together with the Faculty of Medicine, looks back on a rich tradition as the second oldest university medical center in Germany. Today, with around 1,450 beds, the hospital has one of the most modern structural and technical infrastructures in Europe. More than 400,000 inpatients and outpatients are treated here annually at the highest medical level. These benefit from the innovative research power of the scientists, as the latest findings from medical research are quickly and securely transferred into medical practice here.

Muldental Clinics Inc. (non-profit) is the reliable healthcare partner for the people and communities in the Leipzig district. Over 950 employees ensure around-the-clock regional basic and standard care with the disciplines of surgery, internal medicine, gynecology / obstetrics and pediatrics and adolescent medicine with a total of 355 planned beds – ISO-certified and at the cutting edge of research. In addition, the Muldentalkliniken offer recognized special services in hand surgery, tumor surgery and therapy, thyroid surgery and palliative medicine.
The Muldentalkliniken GmbH has its headquarters in Wurzen. The non-profit company of Muldentalkliniken includes the two traditional hospitals in Grimma and Wurzen, the medical care centers (MVZ) in Colditz, Wurzen and Grimma as well as the retirement home company Muldental gGmbH in Wurzen and Brandis. The sole shareholder of the municipal company, which has been successful since 1997, is the district of Leipzig.

Founded in 2007, Xantas AG is an SME that develops software for data analysis in hospitals. The software development center is located in Leipzig. XANTAS mainly produces the software solution VISMEDICA, which is now in use at more than 100 hospitals in Germany, Austria and Switzerland, also thanks to its modular structure. In these hospitals – with a total capacity of more than 60,000 hospital beds – the software is the sound basis for analyses in the areas of medical, surgical or financial controlling of almost 5 million annually treated outpatients and inpatients, among others.
The main focus of the software development so far was on the one hand the connection of SAP-based clinical information systems, on the other hand the creation of classical data warehouse models on the basis of a nomenclature and systematics especially developed for the health care sector. The 10 years of experience in the field of systematization in the analysis process design of a hospital enables the company to react quickly and comprehensively also to new challenges in the healthcare sector and to pursue innovative approaches.
Publications
Sebastian Gibb; Thomas Berg; Adam Herber; Berend Isermann; Thorsten Kaiser (2023): A new machine-learning-based prediction of survival in patients with end-stage liver disease. In Journal of Laboratory Medicine, vol. 47, no. 1, pp. 13-21. https://doi.org/10.1515/labmed-2022-0162
Tom Sicker; Martin Federbusch; Berend Isermann; Wiebke Fenske; Charlotte Fries; Maria Schmidt; Thorsten Kaiser (2023): Challenge in hyponatremic patients – The potential of a laboratory-based decision support system for hyponatremia to improve patient’s safety. In: Clinical chemistry and laboratory medicine 61. https://doi.org/10.1515/cclm-2022-0883
Paul C. Ahrens; Daniel Steinbach; Maria Schmidt; Martin Federbusch; Lara Heuft; Christoph Lübbert; Matthias Nauck; Matthias Gründling; Berend Isermann; Sebastian Gibb; Thorsten Kaiser (2022): Applying Machine Learning to Blood Count Data Predicts Sepsis with ICU Admission. In: medRxiv. https://doi.org/10.1101/2022.10.21.22281348.
David Kotzerke; Maria Walter Costa; Jenny Voigt; Alisa Kleinhempel; Maria Schmidt; Tim Söhnlein; Thorsten Kaiser; Reinhard Henschler (2022): Novelle QLL 2020 – welche Auswirkungen haben die neu empfohlenen Hämoglobin-Transfusionstrigger auf die klinische Versorgung? In: Transfusionsmedizin 12 (01), S. 26–36. https://doi.org/10.1055/a-1669-3918.
Thea Kister; Maria Schmidt; Jenny Voigt; Heike Richter; Michael Haase; Thorsten Kaiser (2022): Diagnosehilfe für akutes Nierenversagen: Laborbasierter Alarm erhöht die Patientensicherheit. In: Deutsches Ärzteblatt 119 (13), S. 567–568. https://www.aerzteblatt.de/archiv/224461/Diagnosehilfe-fuer-akutes-Nierenversagen-Laborbasierter-Alarm-erhoeht-die-Patientensicherheit.
Christiane Gärtner; Romy Langhammer; Maria Schmidt; Martin Federbusch; Kerstin Wirkner; Markus Löffler; Berend Isermann; Ulrich Laufs; Rolf Wachter; Thorsten Kaiser (2021): Revisited Upper Reference Limits for Highly Sensitive Cardiac Troponin T in Relation to Age, Sex, and Renal Function. In: Journal of clinical medicine 10 (23). https://doi.org/10.3390/jcm10235508.
Thea Sophie Kister; Johannes Remmler; Maria Schmidt; Martin Federbusch; Felix Eckelt; Berend Isermann; Heike Richter; Markus Wehner; Uwe Krause; Jan Halbritter; Carina Cundius; Markus Voigt; Alexander Kehrer; Jörg Michael Telle; Thorsten Kaiser (2021): Acute kidney injury and its progression in hospitalized patients-Results from a retrospective multicentre cohort study with a digital decision support system. In: PloS one 16 (7). https://doi.org/10.1371/journal.pone.0254608.
Maria Beatriz Walter Costa; Mark Wernsdorfer; Alexander Kehrer; Markus Voigt; Carina Cundius; Martin Federbusch; Felix Eckelt; Johannes Remmler; Maria Schmidt; Sarah Pehnke; Christiane Gärtner; Markus Wehner; Berend Isermann; Heike Richter; Jörg Telle; Thorsten Kaiser (2021): The Clinical Decision Support System AMPEL for Laboratory Diagnostics: Implementation and Technical Evaluation. In: JMIR Medical Informatics 9 (6). https://doi.org/10.2196/20407.
Georg Schleicher (2021): Assessing the technology and market readiness level of a software system – an analysis based on AMPEL. https://doi.org/10.5281/ZENODO.5763776.
Felix Eckelt; Johannes Remmler; Thea Sophie Kister; Mark Wernsdorfer; Heike Richter; Martin Federbusch; Michael Adler; Alexander Kehrer; Markus Voigt; Carina Cundius; Jörg Telle; Joachim Thiery; Thorsten Kaiser (2020): Verbesserte Patientensicherheit durch „clinical decision support systems“ in der Labormedizin. In: Der Internist 61 (5), S. 452–459. https://doi.org/10.1007/s00108-020-00775-3.
Sebastian Wegener; Felix Eckelt; Martin Federbusch; Carina Cundius; Markus Voigt; Alexander Kehrer; Jörg Telle; Thorsten Kaiser (2019): A Digital Approach to Improve the Management of Patients with Inflammatory Immune Response (AMPEL). In: Journal of Laboratory Medicine, vol. 43. https://doi.org/10.1515/labmed-2019-0135